News & Blogs
In this blog, we touch on diverse topics about Japanese food cultures, practices together with the culinary secret, TREHA®, and its important role in the Japanese food industry. We hope our blog helps you obtain in-depth knowledge of the secrets and science behind Japanese cuisine, shared from our kitchen, to yours.
We interviewed Dr. Suzuki, a specialized professor at Tokyo University of Marine Science and Technology in August, 2022. He has conducted research about food freezing technologies and glass transition behavior of many substances.
Frozen foods are indispensable in our daily lives, and have become popular as early the 1940s in the U.S., and 1960s in other areas, such as Japan. Although we recognize that frozen foods have dramatically changed our food landscape, very few of us know about the science and techniques behind it. In this interview, prof. Suzuki explains the basics of food freezing. What is the definition of being “frozen”, and how can we maintain quality of food after freeze-thaw? Prof. Suzuki talked about freezing from a scientific point of view in this three-part series.
Professor Suzuki’s research – Glass Transition Behavior of Food Ingredients
Team TREHA®: Freezing is one of the hottest trends in food manufacturing in recent years. Could you tell us what has your research been about?
Prof. Suzuki: I have been studying freezing since 1988. My research has moved into phenomenon associated with freezing food ingredients, and assessing practical ways of food freezing. I learned about “glass transition” of substances when I studied in Britain during the initial phase of my research, and it has been extremely important in my career. When food is chilled to below-zero temperature, water becomes ice and other components of food are condensed. Then, food components suddenly become solid if chilling is ongoing – there is a point that condensed viscous liquid changes to solid. This phenomenon is called “glass transition” or “vitrification” and is known as a very important factor in frozen food quality. If the components of food are not vitrified, the molecules keep moving and result in quality loss. Vitrified food components have much less molecular movement, and lead to higher quality during storage. I was convinced that deeply understanding vitrification was beneficial for improving freezing techniques and food quality during storage, and forged ahead in the study of this.
Team TREHA®: We know glass transition as one of the characteristics of trehalose, the main component of TREHA®.
Prof. Suzuki: Yes, you are right. I found many kinds of saccharides which are correlated with vitrification, and trehalose is one of them. Trehalose is one of my focuses, and findings so far are integral to improving food quality; trehalose maintains the quality of fish surimi by suppressing protein denaturation, and helps to restore the crispy texture of frozen deep-fried food when reheated. The texture of food is created by cooking, and saccharides, such as trehalose, facilitate vitrification.
Team TREHA®︎: What kind of research have you been involved in in recent years?
Prof. Suzuki: I have been investigating the mechanisms of texture loss of frozen vegetables. Frozen vegetables lose their texture when thawed, and I have focused on these various phenomenon caused by thawing. I found enzymes are one of the key drivers; enzymatic activity leads to quality loss of the vegetables during thawing. I have been focused on finding the best way to thaw food, and have tried to reveal various mechanisms related to the thawing process.
Column: What is “glass”, a solid generated from liquid at low temperature?
Prof. Suzuki talked about “glass transition” in his talk. But what is the glass, after all?
Glass as for foods is a solid generated from liquid by lowering temperature or by moisture loss. It is different from crystals; crystal is made from molecules aligned in a regular manner; glass is made from random molecular aggregation. You may imagine a glass window when you hear “glass” – and you are right, it is solid made from silica after heating. Food ingredients, such as saccharides and proteins also become a solid, stable glass state.
A good example showing the correlation between frozen food quality and glass transition is frozen tuna. Tuna loses its fresh red color unless it is kept at a very low temperature, such as -60℃/-76℉. Prof. Suzuki reveals how tuna keeps its red color during storage. The component of tuna responsible for its color is condensed by freezing, and is vitrified at a very low temperature; discoloration of tuna is suppressed by glass transition. We can enjoy the fresh color of tuna thanks to this freezing technique in ultralow temperature.
The Process of freezing – What happens when the food freezes?
Team TREHA®: Could you explain what happens inside of food when it is frozen?
Prof. Suzuki: Freezing is the transformation process of moisture from liquid to ice at a low temperature. Water is not always frozen below 0℃/32℉; when this occurs, it is called “freezing-point depression”. You may have studied it in junior high science class by using salt water. Although foods show similar behavior, freezing points differ among foods depending on their composition.
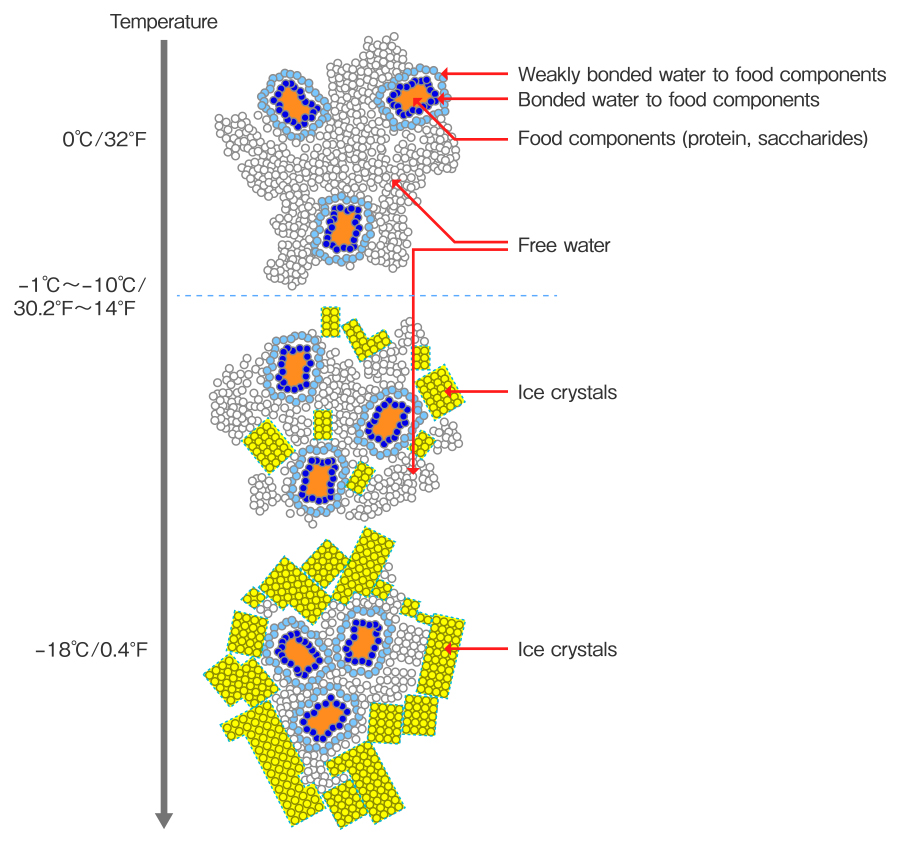
When the temperature of food decreases, free water around the food starts to crystalize. Most of the moisture becomes ice if it continues to chill, and solid content is condensed.(Cited from: Basics of frozen food, Journal of the Japan dietetic association, Vol.61)
Team TREHA®: Does chilling speed have any impact on crystallization?
Prof. Suzuki: Yes it does. Ice needs cores to be formed; you can think of these as “seeds of ice”. Water molecules gather toward the cores and start crystallizing slowly. The state of ice crystals is determined by temperature when freezing. Blast-freezing produces large numbers of small ice crystals, and slow-freezing produces small numbers of large ice crystals. When moisture starts crystallizing, the food is kind of a mixture of ice and concentrated solution that is made from food components and reduced water. If the food passes through this “mixture state” quickly, ice crystals become small and numerous. On the other hand, ice crystals grow bigger and aggregate if the food passes through this “mixture state” slowly.
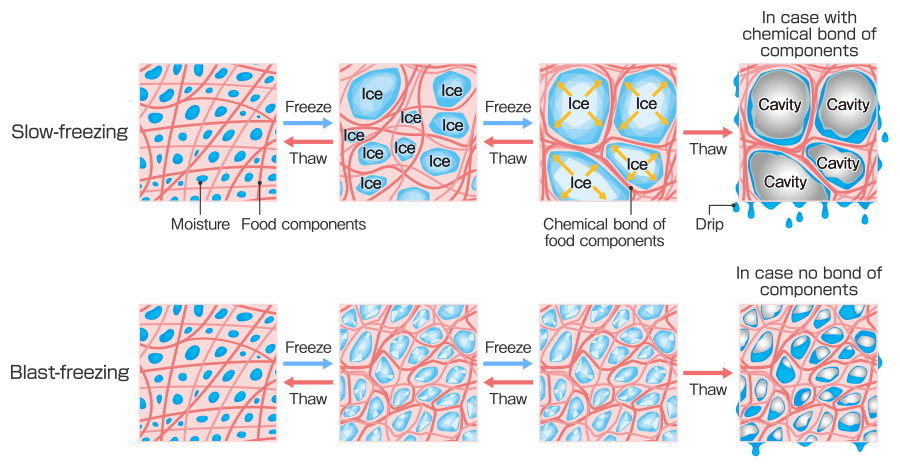
Slow freezing tends to generate large ice crystals and damages surrounding food components. Although it depends on the food components, essentially, solids are aggregated, and moisture drips from the food components when defrosted. In this case, aggregated solids cannot be restored even after thawing, and results in damage to the food structure and an unpleasant texture. On the other hand, blast-freezing makes ice crystal size smaller, and inhibits frozen damage in the food. Structure and texture of food tend to be restored better than slow freezing when defrosted.
The “bonds” among food ingredients – one of the key factors of frozen food’s quality
Team TREHA®: It is said that blast freezing is better than slow freezing to manufacture high quality frozen food. Is this attributed to ice crystal size?
Prof. Suzuki: It is said that blast freezing is better than slow freezing in terms of manufacturing high quality frozen food, because ice crystals generated by blast freezing are smaller than that of slow freezing. Food components tend to be damaged physically by ice crystal growth. However, my research revealed that freezing speed has smaller impact than expected. Although many people believe blast freezing is the ultimate way to maintain frozen food quality, I’d like to say that quality of frozen food is not always determined by freezing speed.
Team TREHA®: I see. What is the key factor for manufacturing high quality frozen food?
Prof. Suzuki: What’s important is the status of the food components other than frozen moisture. I talked about solid concentration during freezing earlier (click here for the previous blog article); the key is the bond among solid food components. If solids in food components are bonded together, moisture cannot reintegrate into food components when thawed. The moisture is isolated from food components and causes syneresis. On the other hand, if solids are not bonded together during freezing, the moisture can reintegrate back into the solids when thawed, so to speak, frozen food is “reconstituted”. Molecular bonds among solids proceed not only during freezing but also in frozen storage. It is important to consider how we can slow down molecular bond formation during freezing and in frozen storage.
Column: Water is not always frozen under zero – Supercooling
Pure water becomes frozen under 0℃/32℉ - do you know that this is not always true? When the water is chilled very slowly, water may maintain a liquid state, even under -10℃/14℉ or -20℃/-4℉. This phenomenon is called “supercooling”, the state of chilling without forming ice cores. Ice crystals cannot be formed and grown without ice cores, as prof. Suzuki explained. Interestingly, this phenomenon cannot be controlled by humans even with modern techniques and has been one of the barriers for precise quality control of frozen food manufacturing.
On today’s blog, prof.Suzuki explained about process of freezing and what glass transition is. On next blog, he will talk about how to maintain the quality of frozen food. What kind of techniques contribute to improved quality of frozen foods? Please stay tuned!
Did you find this blog interesting?
Please share it with your friends in the food service industry.
We regularly update the blog about the food culture of Japan, where TREHA® was discovered for culinary applications.
Click here and send us a message to subscribe.
Or hit us up on Instagram @trehalose_sensei!
You might also be interested in:
Exclusive interview with professor Toru Suzuki, an authority on the phenomenon of freezing and the science of frozen foods:
#1 of 3, #2 of 3, #3 of 3Chef Franck Kestener: The Patisserie Bridging Various Textures with TREHA®
Sugar 101 [Lesson 3] : Protein and Sugar - Coagulation by Heat

